UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 6, 2023
(Exact Name of Registrant as Specified in Its Charter)
| Not applicable | ||||||||
| (State or other Jurisdiction of Incorporation) | (Commission File Number) | (I.R.S. Employer Identification No.) | ||||||
(Address of Principal Executive Offices; Zip Code)
+1 (716 ) 676-6461
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | ||||||||
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | ||||||||
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | ||||||||
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) | ||||||||
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. | ||||
COMPASS Pathways plc (the "Company") is furnishing an updated corporate presentation, attached as Exhibit 99.1 to this Current Report on Form 8-K (the “Corporate Presentation”), which the Company intends to post on the Company’s website and to use from time to time in meetings with investors and others beginning at the 41st Annual J.P. Morgan Healthcare Conference. The Corporate Presentation is current as of January 6, 2023, and the Company disclaims any obligation to update this material in the future.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01. | Financial Statements and Exhibits. | |||||||
| (d) | Exhibits. | ||||
The following exhibits are filed herewith:
| Exhibit No. | Description | |||||||
| 99.1 | ||||||||
| 104 | Cover page interactive data file (embedded within Inline XBRL document) | |||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| COMPASS PATHWAYS PLC | ||||||||||||||||||||
| Date: January 6, 2023 | By: | /s/ Michael Falvey | ||||||||||||||||||
| Michael Falvey | ||||||||||||||||||||
| Chief Financial Officer | ||||||||||||||||||||

Transforming Mental Health Care JANUARY 2023

2 | © COMPASS Pathways plc 2023 Cautionary Note Regarding Forward-Looking Statements This presentation includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. In some cases, you can identify forward-looking statements by terms such as “believe,” “continue,” “could,” “estimate,” “expect,” “may,” “might,” “plan,” “potential,” “project,” “should,” “target,” “will,” “would,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. However, not all forward-looking statements contain these identifying words. These forward-looking statements include express or implied statements relating to our strategic plans or objectives, our plans and expected timing for our phase 3 program in treatment resistant depression and the potential for that or other trials to support regulatory filings and approvals, our plans and expected timing for our phase 2 trials in anorexia nervosa and post traumatic stress disorder, the future accessibility of COMP360 psilocybin therapy, our ability to launch and successfully commercialize COMP360 psilocybin therapy, potential revenue streams if COMP360 psilocybin therapy is approved and our ability to advance COMP360 psilocybin therapy in other areas of high unmet mental health need and to discover and advance new drug compounds. By their nature, these statements are subject to numerous risk and uncertainties, including the impact of global macroeconomic trends on our business, our expectations about the outcomes of our clinical programs, actions of regulatory agencies, our dependence on third parties in connection with our clinical trials and other factors beyond our control, that could cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied in our statements. For additional disclosure regarding these and other risks we may face, see the disclosure contained under the heading "Risk Factors" and elsewhere in the Company’s most recent Quarterly Report on Form 10-Q and subsequent public filings with the US Securities and Exchange Commission (the “SEC”). You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we, nor any other person, assumes responsibility for the accuracy and completeness of these statements. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. Except as required by applicable law, we undertake no obligation to update these forward-looking statements to reflect any new information, events or circumstances after the date hereof, or to reflect the occurrence of unanticipated events. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Market & Industry Data Projections, estimates, industry data and information contained in this presentation, including our general expectations about our market position and market opportunity, are based on information from third-party sources, publicly available information, our knowledge of our industry and assumptions based on such information and knowledge. Although we believe that our third party-sources are reliable, we cannot guarantee the accuracy or completeness of our sources. . All of the projections, estimates, market data and industry information used in this presentation involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such information. In addition, projections, estimates and assumptions relating to our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including, but not limited to, those described above, that could cause future performance to differ materially from our expressed projections, estimates and assumptions or those provided by third parties. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Disclaimer

3 | © COMPASS Pathways plc 2023 We're a mental health care company. We’re committed to developing innovative, evidence-based therapies that help patients and their families, and ease the burden on our overstretched healthcare systems.

4 | © COMPASS Pathways plc 2023 Our synthetic, high-purity polymorphic crystalline formulation of psilocybin, a psychoactive compound. COMP360 psilocybin therapy includes three elements COMP360 psilocybin Psychological support from registered and trained mental health professionals. . Psychological support A patient app, therapist portal and AI-driven analytics platform enhancing patient experience and outcomes. Digital tools COMP360 psilocybin therapy

PLEASE DO NOT DISTRIBUTE 5 | © COMPASS Pathways plc 2023 TRD treatment pathway: significant unmet need for 100 million patients Treatment pathway stage New onset depression Major depressive disorder (MDD) Persistent depression Major depressive disorder (MDD) Treatment-resistant depression (TRD) Line of therapy Estimated number of patients (worldwide) 320 million 200 million 100 million (~1 in 3 of total) US health care cost approx $17-25k per patient/year Available treatments – Antidepressants – Psychological interventions, e.g., CBT* – Antidepressants – Antidepressant combinations – Psychological interventions – Antidepressants – Augmentation therapy (antidepressants, mood stabilizers, anticonvulsants, atypical antipsychotics, esketamine) – Ketamine – Somatic therapy (rTMS, tDCS, ECT, DBS)* – High-intensity psychological interventions % relapse 60-70% 50-75% 80-90% First line Second line Third line + *NOTE: CBT = cognitive behaviorial therapy; rTMS = repetitive transcranial magnetic stimulation; tDCS=transcranial direct current stimulation; ECT=electroconvulsive therapy; DBS=deep brain stimulation SOURCETable adapted from Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., ... & Fava, M. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. American Journal of Psychiatry, 163(11), 1905-1917; Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2):20m13699.

6 | © COMPASS Pathways plc 2023 In a randomized, controlled, double- blind trial, three groups of participants were given a single dose (either 1mg, 10mg or 25 mg) of COMP360 psilocybin alongside psychological support. Results were measured as a change on the MADRS* depression scale from baseline (a day prior to administration) over a 12-week period. The primary endpoint of this study was the change from baseline in MADRS total score at week 3. 1 mg 10 mg 25 mg Rapid onset of action: The effect occurred the day after the administration. Efficacy: We saw a statistically significant and clinically meaningful reduction in depression symptoms. Durability: We saw a sustained response at week 12 – a positive indication for high potential as a monotherapy. Phase 2b trial: Results demonstrate the potential for a rapid, sustained response in TRD NOTE: *Least square mean change from baseline in MADRS total score; MADRS = Montgomery-Åsberg Depression Rating Scale Difference vs 1 mg at Week 3 - 25 mg: -6.6 (95% confidence interval: -10.2 ; -2.9), p<0.001 - 10 mg: -2.5, p=0.184 Primary efficacy assessment, 3 weeks post administration Follow-up period, 3-12 weeks post administration Published in The NEW ENGLAND JOURNAL of MEDICINE

7 | © COMPASS Pathways plc 2023 Sustained responders are participants who responded (≥50% change in MADRS total score from baseline) at weeks 3 and 12, and at least one visit out of week 6 and 9, and who did not start new treatments for depression. Sustained non-responders are participants who did not respond (<25% change in MADRS total score from baseline) at weeks 3 and 12, and at least one visit out of week 6 or 9. Quality of life: Sustained responders were found to have a clinically meaningful increase in quality of life from baseline at week 3 and week 12 with scores in the normal range after treatment Positive affect: Sustained responders were found to have a clinically meaningful increase in positive affect from baseline on the day after the psilocybin session and at week 3 Phase 2b trial: Those participants who showed a sustained response also showed signs of improvement beyond the reduction of depression symptoms NOTE: EQ-5D-3L= EuroQoL 5-Dimensions 3-Levels; PANAS= Positive and Negative Affect Schedule; SD= standard deviation Sustained responders (n=19) Sustained non-responders (n=21)

8 | © COMPASS Pathways plc 2023 There were no concerns with vital signs, ECG or clinical laboratory data in any of the treatment groups TEAEs involving hallucinations (which only occurred in the 25mg and 10mg groups) and illusions (all groups) started and resolved on the day of administration. TEAEs of suicidal ideation, suicidal behavior and intentional self-injury were seen in all groups, as is regularly observed in a TRD population. – All patients who experienced these events during the trial had said during screening that they had had suicidal thoughts prior to the trial. – Case-by-case analysis of safety data found no evidence to suggest a causal relationship between these TEAEs and administration of COMP360 psilocybin. The majority occurred more than a week after the psilocybin session. >90% of TEAEs were of mild or moderate severity. 5 most frequent TEAEs across the 10mg and 25mg doses were headaches, nausea, fatigue, insomnia and anxiety. Treatment-emergent adverse events (TEAEs) >77% of TEAEs occurring on the day of administration resolved on the same or next day; most were mild or moderate. Phase 2b trial: COMP360 psilocybin therapy was generally well-tolerated

9 | © COMPASS Pathways plc 2023 Pivotal trial 1 Single dose monotherapy (COMP 005) COMP360 25 mg Placebo Pivotal trial 2 Fixed repeat dose monotherapy (COMP 006) COMP360 25 mg COMP 360 1 mg COMP360 25 mg COMP360 1 mg COMP360 10 mg COMP360 10 mg Randomisation = 2:1:1 n = 568 (284:142:142) Week 6 Primary endpoint* Randomisation = 2:1 n = 378 (252:126) Week 3 Phase 3 program: Overview of pivotal trial designs *Primary endpoint - change from baseline in MADRS total score at Week 6 The participant population (TRD definition and core inclusion/exclusion criteria) remains unchanged compared to Phase 2b Week 6 Primary endpoint* Long-term follow-up Long-term follow-up Phase 3 program status: Commenced patient recruitment The phase 3 program will be conducted across 150 sites in 14 countries and will include a long-term follow-up component Top line data expected end 2024 Top line data expected mid 2025

10 | © COMPASS Pathways plc 2023 Our offering We will deliver COMP360 (medicine) to treatment centers through speciality pharmacy channels. We will offer training, site activation services and digital solutions to treatment centers. Our revenue streams When reimbursed by payers, we will sell COMP360 (medicine) to specialty pharmacy. We’re assessing the potential for additional revenue streams from licensing our training and digital solutions to treatment centers. Patients Treatment centers and healthcare professionals Speciality Pharmacy Payers Other partners (e.g., training and service delivery) 1 2 3 4 1 1 2 2 3 3 4 Our initial launch model 2
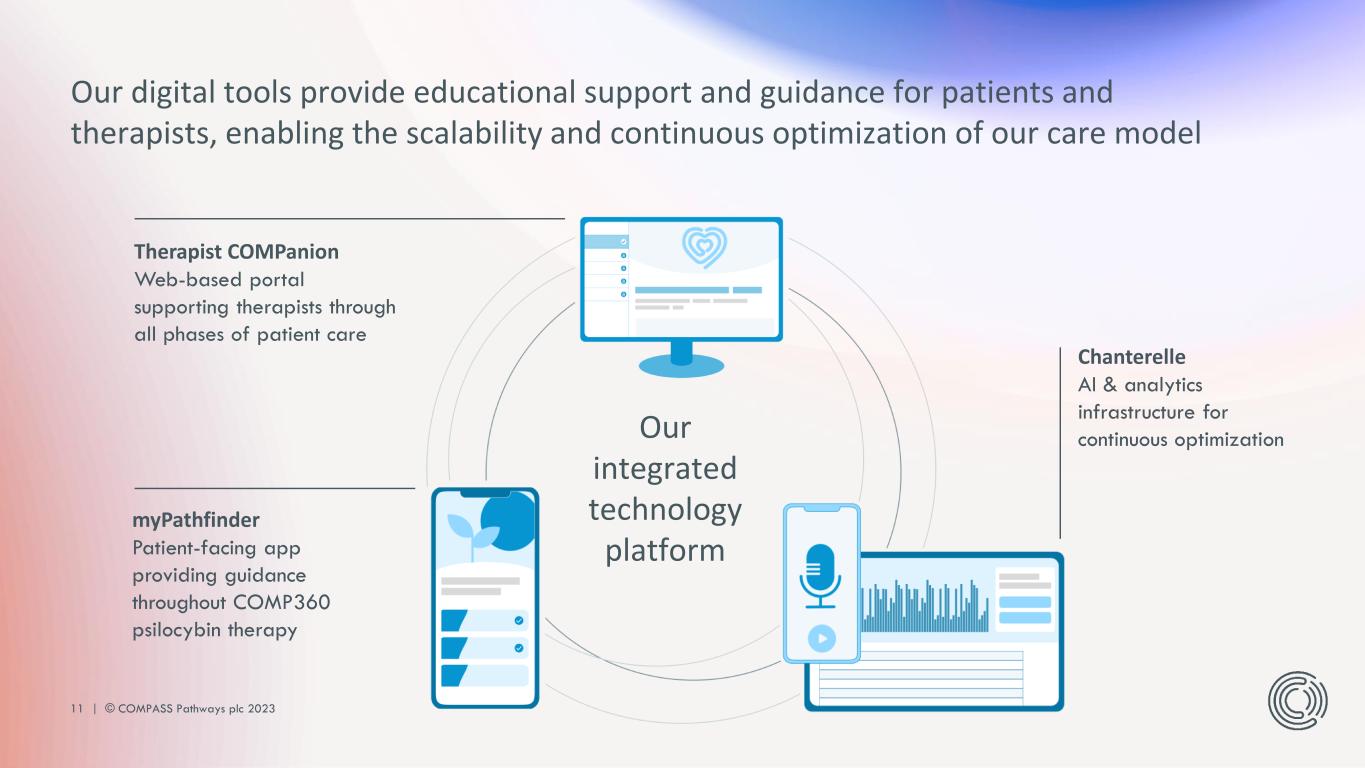
11 | © COMPASS Pathways plc 2023 Therapist COMPanion Web-based portal supporting therapists through all phases of patient care Chanterelle AI & analytics infrastructure for continuous optimization myPathfinder Patient-facing app providing guidance throughout COMP360 psilocybin therapy Our digital tools provide educational support and guidance for patients and therapists, enabling the scalability and continuous optimization of our care model Our integrated technology platform

12 | © COMPASS Pathways plc 2023 Discovery Preclinical Phase 1 Phase 2 Phase 3 Approved COMP360 for TRD COMP360 for anorexia nervosa COMP360 for PTSD Prodrug development Discovery Center (NCE development) Beyond COMP360 psilocybin: We’re investigating prodrugs and novel psychedelic and non- psychedelic chemical entities. Beyond TRD: We’re assessing the safety and efficacy of COMP360 psilocybin therapy for anorexia nervosa and PTSD. We’re continuing to develop a balanced and differentiated pipeline NOTE: NCE = new chemical entity; PTSD = post-traumatic stress disorder; TRD = treatment-resistant depression

13 | © COMPASS Pathways plc 2023 Listed here are signal-generating studies looking at indications in areas of serious unmet need with COMP360 psilocybin. These studies may provide signals for new potential indications for COMP360 psilocybin that we can explore further and bring into our development pipeline. COMPASS owns or has a license to new IP generated around COMP360 psilocybin. Indication Institution Status MDD in cancer patients Aquilino Cancer Center MDD University of Zurich Chronic cluster headache University of Copenhagen Severe TRD Sheppard Pratt Anorexia nervosa UC San Diego Bipolar disorder II Sheppard Pratt Body dysmorphic disorder Columbia University Suicidal ideation Sheppard Pratt Autism King’s College London* TRD Stanford Rumination Massachusetts General Hospital Complete Ongoing We provide support to research institutions conducting investigator-initiated studies with COMP360 psilocybin NOTE: MDD = major depressive disorder ; *A research scientist employed by COMPASS Pathways who is a PhD student at King’s College London is conducting the study

PLEASE DO NOT DISTRIBUTE 14 | © COMPASS Pathways plc 2023 1. Kabir Nath Chief Executive Officer 2. Dr Guy Goodwin Chief Medical Officer 3. Trevor Mill Chief Development Officer 4. Anne Benedict Chief People Officer 5. Mike Falvey Chief Financial Officer 6. Marco Mohwinckel Chief Commercial Officer 7. Matt Owens General Counsel and Chief Legal Officer 8. Greg Ryslik Executive Vice President, AI, Engineering, Digital Health Research & Technology 9. Ekaterina Malievskaia MD Chief Innovation Officer and Co-founder 10. George Goldsmith Chairman and Co-founder 1 2 3 4 5 6 7 8 9 10 We have a team of experts and leaders with a record of delivering visionary innovation in pharma and beyond 3

15 | © COMPASS Pathways plc 2023 Cash and cash equivalents $173.1 million Issued shares 42.5 million Covering analysts – Berenberg, Caroline Palomeque – BTIG, Robert (Bert) Hazlett – Canaccord Genuity, Sumant Kulkarni – Cantor Fitzgerald, Charles Duncan – CITI, Neena Bitritto-Garg – Cowen, Ritu Baral – EvercoreISI, Josh Schimmer – HC Wainwright & Co, Patrick Trucchio – Loop Capital, Esther Hong – Maxim Group, Jason McCarthy – Oppenheimer, Francois Brisebois – ROTH, Elemer Piros COMPASS Financial Overview NOTE: Cash and cash equivalents at September 30, 2022. Issued shares as of 3 November 2022

16 | © COMPASS Pathways plc 2023 We're a mental health care company. – Lead product candidate: COMP360 psilocybin therapy – Phase 2 TRD program published in The New England Journal of Medicine – Phase 3 TRD program expected to commence by end of 2022 • Trial 1: top-line data expected end of 2024 • Trial 2: top-line data expected mid-2025 – Phase 2 anorexia nervosa study - data expected late 2023 – Phase 2 PTSD study – data expected late 2023 – IIS programs expected to generate data

17 | © COMPASS Pathways plc 2023 Appendix

PLEASE DO NOT DISTRIBUTE 18 | © COMPASS Pathways plc 2023 Most frequent TEAEs ordered by the 25mg arm (at least 5% in any treatment group) TEAE incidence is higher in the 25mg group overall Key mood-related TEAEs (euphoric mood, depression, depressed mood, suicidal ideation) do not have a higher incidence in the 25mg arm Note: MedDRA = Medical Dictionary for Regulatory Activities; TEAE = treatment emergent adverse event; N = number of participants in the population; n = number observed MedDRA TEAE preferred term COMP360 25mg COMP360 10mg COMP360 1mg Overall N=79 N=75 N=79 N=233 n (%) Headache 27 (34.2) 16 (21.3) 20 (25.3) 63 (27.0) Nausea 18 (22.8) 7 (9.3) 4 (5.1) 29 (12.4) Fatigue 12 (15.2) 5 (6.7) 7 (8.9) 24 (10.3) Insomnia 8 (10.1) 11 (14.7) 14 (17.7) 33 (14.2) Anxiety 7 (8.9) 13 (17.3) 3 (3.8) 23 (9.9) Mood altered 7 (8.9) 3 (4.0) 1 (1.3) 11 (4.7) Back pain 6 (7.6) 0 3 (3.8) 9 (3.9) Dizziness 6 (7.6) 1 (1.3) 1 (1.3) 8 (3.4) Suicidal ideation 5 (6.3) 5 (6.7) 4 (5.1) 14 (6.0) Myalgia 5 (6.3) 2 (2.7) 1 (1.3) 8 (3.4) Euphoric mood 4 (5.1) 5 (6.7) 4 (5.1) 13 (5.6) Depression 4 (5.1) 6 (8.0) 5 (6.3) 15 (6.4) Abdominal pain upper 4 (5.1) 2 (2.7) 1 (1.3) 7 (3.0) Irritability 4 (5.1) 2 (2.7) 1 (1.3) 7 (3.0) Panic reaction 4 (5.1) 1 (1.3) 1 (1.3) 6 (2.6) Depressed mood 3 (3.8) 5 (6.7) 4 (5.1) 12 (5.2) Paraesthesia 3 (3.8) 4 (5.3) 1 (1.3) 8 (3.4) Thinking abnormal 0 4 (5.3) 0 4 (1.7)

PLEASE DO NOT DISTRIBUTE 19 | © COMPASS Pathways plc 2023 Modulation of cortical and limbic systems via 5- HT2A receptors Note: understood mechanism of action based on studies of psilocybin (not COMP360); *5-HT2A = 5- hydroxytyryptamine 2A; DMN = default mode network; mPFC = medial prefrontal cortex Source: 1. Halberstadt et al (2011); 2. Lopez-Gimenez et al (2018); 3. Vollenweider et al (1999); 4. Sakashita et al (2015); 5. Carhart- Harris et al (2012a); 6. Petri (2014); 7. Ly et al (2018) Prefrontal cortex Posterior cingulate cortex Cortical system Amygdala Hippocampus Limbic system Pyramidal neuron Psilocin (metabolite of psilocybin) 5-HT2A receptor* NH OH N 1. Stimulation of 5-HT2A receptors1 results in downstream cascades via G-protein signalling2. 2. Altered extracellular release of dopamine3,4 and leading to enhanced positive mood. 3. Downregulation of the DMN5, and de- synchronisation of cortical activity as well as the emergence of new patterns of functional connectivity across the brain6. 4. Sustained cellular changes leading to neuroplasticity7 and “window of opportunity” for therapy. Psilocybin mechanism of action
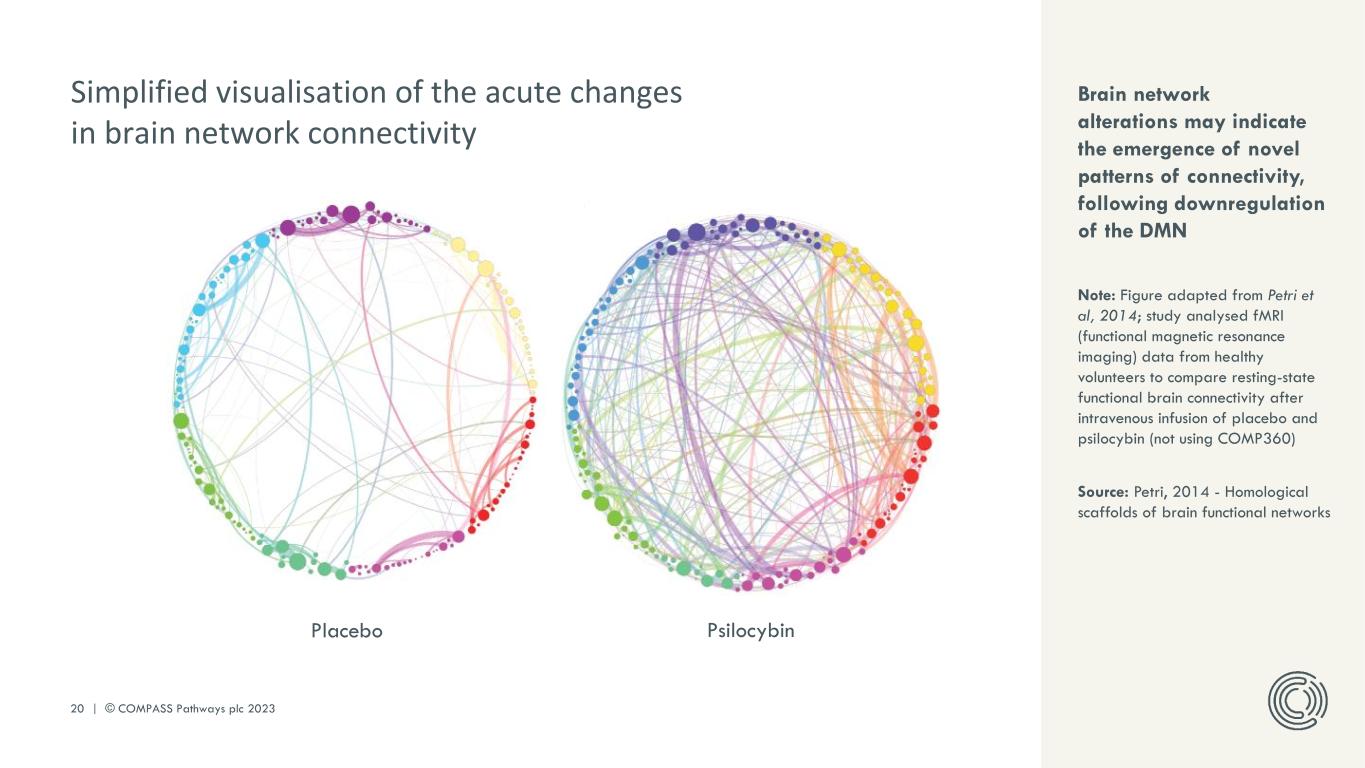
PLEASE DO NOT DISTRIBUTE 20 | © COMPASS Pathways plc 2023 Placebo Psilocybin Brain network alterations may indicate the emergence of novel patterns of connectivity, following downregulation of the DMN Note: Figure adapted from Petri et al, 2014; study analysed fMRI (functional magnetic resonance imaging) data from healthy volunteers to compare resting-state functional brain connectivity after intravenous infusion of placebo and psilocybin (not using COMP360) Source: Petri, 2014 - Homological scaffolds of brain functional networks Simplified visualisation of the acute changes in brain network connectivity

PLEASE DO NOT DISTRIBUTE 21 | © COMPASS Pathways plc 2023 Higher proportion of sustained responders found in the 25mg vs 1mg arm. Note: MADRS = Montgomery- Åsberg Depression Rating Scale; Statistical significance cannot be claimed on secondary endpoints due to hierarchical testing being broken for the 10mg vs 1mg dose on the primary endpoint Participants who started new treatment for depression were assumed to be a non-responder hence decreasing numbers reflecting antidepressant use over time 0 5 10 15 20 25 30 Week 12 COMP360 25mg COMP360 10mg COMP360 1mg 10.7% 10.1% % s us ta in e d r e sp o nd e rs 24.1% Sustained responder – participant meeting the MADRS response criteria at any visit up to and including week 3 and also at week 12 and at least one visit out of week 6 and week 9, and who did not start any new treatments for depression MADRS sustained responders at week 12

22 | © COMPASS Pathways plc 2023 Therapist training model Phase III delivery Value Tier I Self-paced learning Therapist COMPanion platform in a new, dynamic digital format Optimized user learning experience Analytics to ensure learning objectives are achieved Tier II Interactive clinical skills training Therapist COMPanion platform ~8-15 therapists / cohort Optimized delivery of our interactive training online Increased number of groups being trained simultaneously; modular format to facilitate access Tier III Clinical observation training Mixed method training approach: - 2 in-person sessions - 2 recordings of Phase IIb psilocybin sessions Exposure to a range of experiences in supporting patients Gaining confidence through in-person sessions Tier IV Continuing professional development Mentoring delivered online on Therapist COMPanion platform New quality oversight system

23 | © COMPASS Pathways plc 2023 Phase 3 investigational sites COMP 005 COMP 006 US (33) US (43) DEU (7) UK (14) CAN (8) AUS (3) DEN (1) NOR (1) CZR (8) SWE (5) NDL (3) FRA (6) IRE (2) ESP (10) POL (6) New site clustersPhase 2b sites Number of sites per study/country

24 | © COMPASS Pathways plc 2023 • Targeting networks of commercial treatment centers with the right infrastructure, capabilities / workforce and TRD patient mix / flow (eg. Greenbrook TMS, others) • Hundreds of clinics managing tens of thousands of TRD patients • Offering TMS, IV Ketamine, SPRAVATO®, ECT • Able to handle complex delivery, billing and reimbursement • Digitally progressive • Converting Phase III academic centers offering clinical services • Activating new TRD referrals through hub-and-spoke model (already being deployed in Phase III) • Working on establishing new billing codes for new medical services, eg. psychological support during administration • Building training, enabling services and solutions to facilitate clinical adoption and scalability (eg. remote vs. face-to-face, and train-the-trainer models) • Setting up research partnerships with clinic and integrated delivery networks to test lean and scalable delivery models (e.g. simultaneous administration) • Ongoing engagement with commercial sites to assess needs and research collaboration opportunities Ketamine treatment centers Our primary delivery partners will be specialized interventional psychiatry treatment centers Hub and spoke model

PLEASE DO NOT DISTRIBUTE 25 | © COMPASS Pathways plc 2023 COMP360 IP protection and regulatory exclusivity IP protection COMPASS has US patents covering COMP360 (including composition of matter, formulation, methods of treatment, and methods of manufacture) that expire in 2038 (20-year term) as well as pending patent applications covering COMP360 in major markets such as US, UK, and EU. COMPASS to seek Patent Term extension and Supplementary Protection Certificates, where available, that may extend the term of patents that cover the approved product potentially up to five years depending on the date of regulatory approval and patent grant date. A third party challenged the validity of three US patents (US 10,519,175; US 10,647,257; and US 10,954,259) at the USPTO. The USPTO has denied institution of all three challenges, upholding the validity of these patents. Regulatory exclusivity Upon approval – US: Benefit of 5 years New Chemical Entity (NCE) protection. – EU: Benefit of 8+2 years New Active Substance (NAS) protection.

26 | © COMPASS Pathways plc 2023 We anticipate treatment centers will offer COMP360 psilocybin therapy alongside other interventional psychiatry services References: [1] ICER, 2019; [2] Ross, 2018; [3] Petrides, 2011; [4] Thirthalli, 2020; [5] Voigt, 2017 (r)TMS 1h once /day for 30-40 days + screening, education, evaluation Patient time: 20-30h HCP time: 40-50h IV Ketamine 2h infusion, 12-15 sessions over 6 months +, education (and therapy) Patient time: 30-50h HCP time: 50-70h Esketamine 3h (including observation); twice weekly for 1month, then (bi-weekly) Patient time: 90-160h HCP time: 120-240h ECT 4h procedure under general anaesthetic, 6-12 sessions Patient time: 30-50h HCP time: 80-160 COMP360 Episodic, less invasive, and lower burden than alternative interventions 8h administration; est. 1-3 administrations provided over 6 months Patient time: 20-40h HCP time: 20-50h

PLEASE DO NOT DISTRIBUTE 27 | © COMPASS Pathways plc 2023 Anorexia nervosa (AN) AN is an eating disorder characterized by weight loss or difficulties maintaining a healthy body weight, usually associated with distorted body image. People with AN generally restrict their caloric intake, types of food they eat, and might engage in purging behaviors (eg, strenuous exercise, vomiting, laxatives/diuretics misuse). 3.9M people suffer with AN; it has a lifetime prevalence up to 4% in females. 20% of deaths in AN are due to suicide; it’s the deadliest of psychiatric disorders. 0 no pharmacological treatments approved; psychological treatments have relapse rates as high as 52%. SOURCE: Our World In Data; NICE, 2019; Khalsa et al., 2017 Where we are at… Secondary endpoints Change in obsessive-compulsive symptoms and change in weight at week 12 Latest trial (ongoing) P2a to determine proof of concept in AN 60 participants Multi-national, multi-center, randomized, double-blind study Single dose of 25mg COMP360 psilocybin vs 1mg administered with psychological support Primary endpoint AN symptoms reduction

PLEASE DO NOT DISTRIBUTE 28 | © COMPASS Pathways plc 2023 Where we are at… Post-traumatic stress disorder (PTSD) PTSD can occur in people who have experienced or witnessed a traumatic event (eg, natural disaster, serious accident, war, rape). Some people with PTSD experience symptoms from immediately after the event while for others symptoms may appear years later. 311M People will experience PTSD at some point in their lives. 20-30% of patients treated with currently approved pharmacological interventions for PTSD will reach full remission. $17K Direct medical costs per patient per year in a large veteran population in the US. SOURCE; Kautz et al., 2017; Kessler et al., 2005; Van Ameringen et al., 2008; Koenen et al., 2017; Berger et al., 2009; Wang et al., 2016 NOTE: SSRI = selective serotonin reuptake inhibitor; FDA = Food and Drug Administration Latest trial P2 (ongoing), 20 participants Multi-national, multi-center, open label study Single dose of 25mg COMP360 psilocybin administered with psychological support Primary endpoint Safety and tolerability Secondary endpoints Symptoms reduction, functionality, quality of life, response and remission

29 | © COMPASS Pathways plc 2023 We're a mental health care company. We’re committed to developing innovative, evidence-based therapies that help patients and their families, and ease the burden on our overstretched healthcare systems. Stephen Schultz SVP, Investor Relations stephen.schultz@compasspathways.com +1 401-290-7324
